Abstract
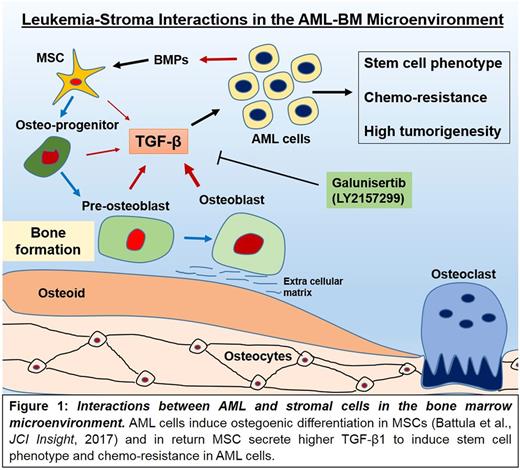
Bone marrow (BM) microenvironment in acute myeloid leukemia (AML) consists of various cell types including mesenchymal stromal cells (MSCs), osteoprogenitor cells, osteoblasts, osteocytes, endothelial, immune and perivascular reticular cells that support AML cell growth and protect them from chemotherapy. We have recently reported that AML cells alter the BM microenvironment by inducing osteogenic differentiation in MSCs to gain growth advantage in the bone marrow (Battula et al, JCI Insight, 2017). However the stroma-induced functional alterations in AML cells are not well understood. Stromal cells induce chemo-resistance in AML cells and as chemo-resistance and high tumorigenic potential are the characteristics of leukemia stem cells, we hypothesize that stromal cells can induce stem cell characteristics in AML cells. To test our hypothesis we co-cultured AML cell lines including OCI-AML3 and HL60 cells with BM-derived MSCs for 3 or 5 days and determined aldehyde dehydrogenase (ALDH) enzyme activity (a well-established marker of hematopoietic and leukemia stem cells) and gene expression by RNA sequencing. We found that the percentage of ALDH+ cells in OCI-AML3 increased from 7% to 89.1% in 3 days and from 23% to 92.3% in 5 days of co-culture with MSCs. Similarly, ALDHwas increased in HL60 cells from 7% to 68% in 3 days and from 20.7% to 67.9% in 5 days of co-culture with MSCs suggesting that stromal cells can induce certain stem cell features in AML cells.
Next, to better determine the mechanism, OCI-AML3 cells were co-cultured with BM-MSCs (isolated from 3 different donors) for 5 days, the OCI-AML3 cells were FACS sorted and gene expression was analyzed by RNA sequencing. Using R statistics we identified differentially expressed genes in OCI-AML3 cells and found that gene sets associated with stem cell function including ALDH2, Wnt5b, Wnt5a were up-regulated whereas inflammatory and innate immune response genes were down-regulated in OCI-AML3 cells co-cultured with MSCs compared to cells cultured alone. These genes were validated by qRT-PCR assay. Ingenuity pathway analysis® (IPA) revealed a TGFβ1 activated gene signature in OCI-AML3 cells co-cultured with MSCs compared to cells culture alone. To investigate the role of TGFβ1 in the stroma-induced stem cell phenotype in AML cells, OCI- AML3 and HL60 cells were treated with recombinant TGFβ1 (5ng/ml) for 3 or 5 days. Indeed, treatment with TGFβ1 induced ALDH positive cells from 27.7% to 89.5% in 3 days and from 3.4% to 92.5% in 5 days (OCI-AML3). Similarly, in HL60 cells treated with TGFβ1, ALDH+ cells increased from 9.8% to 25.1 in 3 days suggesting that TGFβ1 induces a stem cell phenotype in AML cells. Next, we measured levels of TGFβ1 in cell culture supernatants from MSCs by ELISA and found that MSCs secrete TGFβ1 up to 1ng/ml into the surrounding medium. In addition, to study the role of TGFβ1 in stroma-induced stem cell activity in AML cells, we treated AML cells with or without TGFβ1 type-1 receptor kinase inhibitor Galunisertib (LY2157299 monohydrate from Eli Lilly®) in the presence or absence of MSCs. Strikingly, treatment of galunisertib at 1µM completely inhibited MSCs-induced ALDH activity in OCI-AML3 and HL60 cells in 5 day co-culture setting. To determine if galunisertib sensitizes AML cells to chemotherapy in the presence of MSCs, we treated OCI-AML3 with galunisertib (1µM) or cytarabine (1µM) or in combination for 72hrs in the presence or absence of MSCs. As expected presence of MSCs inhibited cytarabine induced cell killing (positive for annexin-V and DAPI) from 47.1% to 28.3% cells. In turn, the combination of cytarabine and galunisertib negated this affect and induced cell killing in OCI-AML3 cells from 28% to 54% even in the presence of MSCs suggesting complete loss of MSC-mediated chemo-protection in OCI-AML3 cells upon inhibition of TGF-β signaling. In-vivo testing of galunisertib in combination with cytarabine and doxorubicin in AML PDX models in NSG mice is currently ongoing.
Collectively, data indicate that stromal cells induce chemo-resistance in AML cell by inducing stem cells phenotype through TGF-beta mediated signaling (Fig 1). TGF-β inhibition by galunisertib, an agent in early clinical trials in solid tumors inhibits stem cell phenotype and BM microenvironment-induced chemo-resistance and facilitates cell killing of AML cells.
Battula: United Therapeutics Corporation: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal